Describe the Electron Density Around Electrophiles
NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless. Electrophiles are electron poor therefore are electron-loving compounds.
Nucleophiles And Electrophiles Organic Chemistry Tutor
Also called Lewis acid because they accept electrons.

. Nucleophiles are electron rich. Electrophiles are those substances that lack electron density electro electron phile lover electron loving species So A is the correct answer. In general the electron is more likely to be found in regions with high electron density.
Weak bases are good leaving groups. Bond Polarity Allows the Bond to be Broken Easier. They are therefore vulnerable to attack by species which are attracted to high electron density.
The electron density around electrophiles is. So an electrophile is electron-loving and since electrons are negatively charged were gonna think about an electrophile as having a region of low electron density so it could have a full positive charge on it because a positive charge would be attracted to electrons or you could be talking about a partial positive charge. Next we turn to electrophiles.
It is a reagent that is characterized with a low density of electrons in its valance shell and therefore reacts with a high-density molecule ion or atom to form a covalent bond. Strong acids are good leaving groups. NCERT P Bahadur IIT-JEE Previous Year Narendra Awasthi MS Chauhan.
The important molecule has a region of high electron density which is attacked by something carrying some degree of positive charge. An electrophilic addition reaction is an addition reaction which happens because what we think of as the important molecule is attacked by an electrophile. Consequently electrophiles and nucleophiles are species in organic chemistry that interact very much like acids and bases.
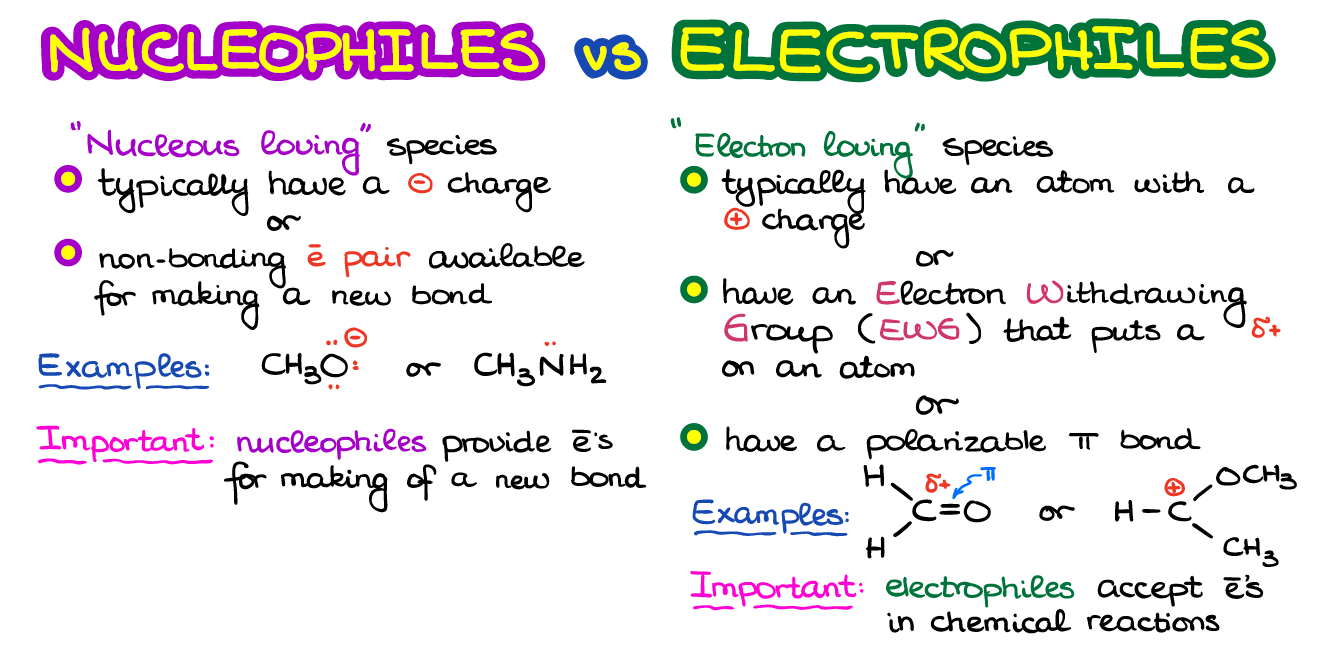
A species that is attracted to the nucleus of another atom. Because electrophiles accept electrons they are Lewis acids. Favour electrophilic addition and electrophilic substitution reactions.
Since they accept electrons they are also known as Lewis acids. In chemistry an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. They attack electron rich parts of molecules.
Weak acids are good leaving groups. The important molecule has a region of high electron density which is attacked by something carrying some degree of positive charge. Electron density is a representation of the probability of finding an electron in a specific location around an atom or molecule.
The π bond electrons in the electron tend to have a high electron density making it easy to polarise give up electrons and behave as a nucleophile. The concept of electrophilicity is relatively simple. Electrophiles mainly interact with.
Brønsted and Lowry have given us a simple definition of acids and bases. In electrophilic substitution and addition reactions electrophiles are involved. Learn about the definition of electrophiles the electrophiles-nucleophile relationship the electrophilic addition reaction and see some examples of electron-rich sites in organic molecules.
C-C sigma bond C-C p bond Electrophiles tend to be molecules where part of the molecules has a slight positive charge. An acid is a proton donor and a base is a proton acceptor. The density influences electron movement which generally occurs from a high-density to a low-density region.
Solve any question of Organic Chemistry - Some Basic Principles and Techniques with-. They could be called electron deficient. An electrophilic addition reaction is an addition reaction which happens because what we think of as the important molecule is attacked by an electrophile.
These species are called electrophiles. Consider electron density distribution. Electrophiles will often have electron-withdrawing groups a group containing electronegative elements pulling the electron density towards themselves.
This behaviour is remarkably similar to that of lone pair electrons on a Lewis base. Electrophilic addition and electrophilic replacement reactions should be favored. By measuring the electron density around the nucleus it is possible to define regions where electrons are most likely to be found at.
Hydrogen ion in acids and methyl-carbocation are examples of electrophilic substances. So lets say this is your nucleus and then the block dots are where electrons could be. So if years the squared wave function do you produce an electron density diagram then I dont tell you how likely it is that electrons or units are in area or the probability density.
Movement of electrons is affected by the density and the movement is generally from a high-density area to low-density area. Alternatively electrophiles may also have polarizable π-bonds such as CO or CN. An electron behaves as though it is spread out around the nucleus as a sort of cloud sometimes referred as an electron cloud.
For example in the picture at the beginning of this post we have a couple of electrophilic molecules. In simple terms it means electrons-loving. Electrophiles are involved in electrophilic substitution and addition reaction.
Electrophiles are electron deficient and attack the substrate where the electron density is more. And electrophiles are positively charged species. In the vast majority of the nucleophilic substitution reactions you will see in this and other organic chemistry texts the electrophilic atom is a carbon bonded to an electronegative atom usually oxygen nitrogen sulfur or a halogen.
So an electron density diagram looks something like this. Most electrophiles are positively charged have an atom that carries a partial positive charge or have an atom that does not have an octet of electrons. They are electron deficient.
-an electron-withdrawing group is attach to the conjugate base-the size of the conjugate base increases-the electronegativity of the atom carrying a negative charge in the conjugate base increases-as the electron density of the conjugate base decreases its stability increases. However due to the uncertainty principle its not possible to identify the exact location of an electron at any instant in time. Then the areas that have more dense dots are the areas.
Lewis introduced the terms nucleophile and electrophile to describe Lewis acids and bases. Lewis acids are those substances that can accept a lone pair of electrons from an electron rich species Electrophiles are those substances that lack electron density electro electron phile lover electron loving species So B is the correct answer. Strong bases are good leaving groups.
1 not relevant for electrophiles 2 relatively low 3 relatively high 4 unaffected 15Which description about leaving groups is correct 1. Electrophiles are electron deficient and attack the substrate where the electron density is more.
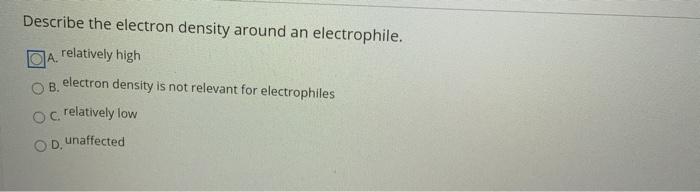
Solved Describe The Electron Density Around An Electrophile Chegg Com

Solved 14 The Electron Density Around Electrophiles Is 1 Chegg Com

Solved Describe The Electron Density Around An Electrophile Relatively High Electron Density Is Not Relevant For Electrophlles Crelatively Low Unaffected
No comments for "Describe the Electron Density Around Electrophiles"
Post a Comment